Chlorine Electronegative Than Carbon . the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. examples include most covalent bonds. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to.
from www.numerade.com
provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. examples include most covalent bonds. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale.
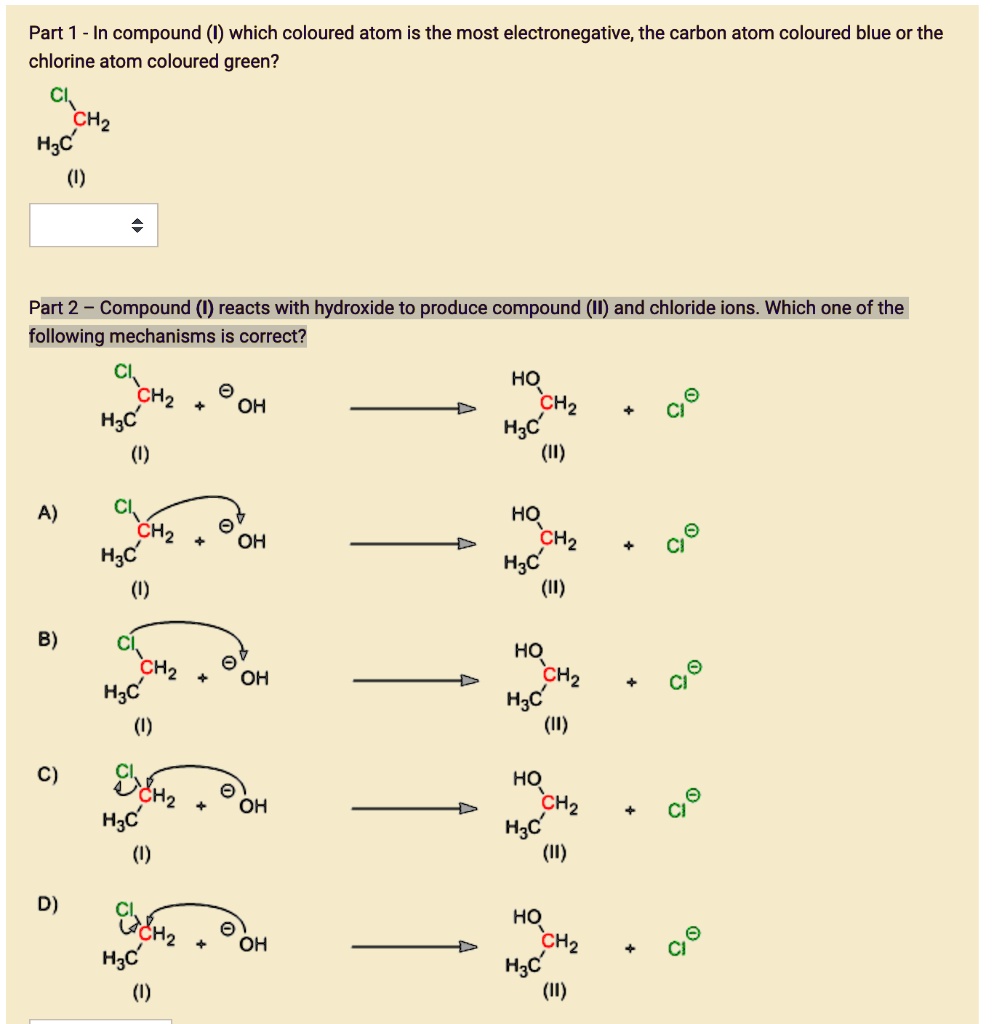
SOLVED Part 1 In compound (V) which coloured atom is the most
Chlorine Electronegative Than Carbon the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. examples include most covalent bonds. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to.
From mungfali.com
Atom Electronegativity Chart Chlorine Electronegative Than Carbon 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. examples include most covalent bonds. since chlorine is about as electronegative as nitrogen, the effect. Chlorine Electronegative Than Carbon.
From www.numerade.com
SOLVEDJudging from their relative positions in the Periodic Table Chlorine Electronegative Than Carbon the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a. Chlorine Electronegative Than Carbon.
From www.youtube.com
The most electronegative element in the periodic table is chlorine Chlorine Electronegative Than Carbon provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. examples include most covalent bonds. since chlorine is about as electronegative as nitrogen, the effect of. Chlorine Electronegative Than Carbon.
From brokeasshome.com
Periodic Table Chlorine Electrons Chlorine Electronegative Than Carbon since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. examples include most covalent bonds. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. 119 rows electronegativity is a chemical property which. Chlorine Electronegative Than Carbon.
From www.vedantu.com
With respect of chlorine, hydrogen will be(A) Electropositive(B Chlorine Electronegative Than Carbon since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. provided that you understand what happens when electronegative atoms like fluorine or. Chlorine Electronegative Than Carbon.
From www.expii.com
Polar vs. Nonpolar Bonds — Overview & Examples Expii Chlorine Electronegative Than Carbon provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and. Chlorine Electronegative Than Carbon.
From byjus.com
why flourine is most electronegative?why not chlorine Chlorine Electronegative Than Carbon the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. examples include most covalent bonds. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. since chlorine is about as electronegative as nitrogen, the effect of. Chlorine Electronegative Than Carbon.
From wisc.pb.unizin.org
Molecule Polarity (M9Q2) UWMadison Chemistry 103/104 Resource Book Chlorine Electronegative Than Carbon the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. since chlorine is about as electronegative as nitrogen, the effect of a chlorine. Chlorine Electronegative Than Carbon.
From www.nagwa.com
Question Video Identifying Which Silicon Electronegativity Comparison Chlorine Electronegative Than Carbon provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. the two chlorine atoms share the pair of electrons in the single covalent bond equally,. Chlorine Electronegative Than Carbon.
From ecurrencythailand.com
When An Atom Of Chlorine Forms An Ion The Atom? The 8 New Answer Chlorine Electronegative Than Carbon since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. provided that you understand what happens when electronegative atoms like fluorine or chlorine are. Chlorine Electronegative Than Carbon.
From whatsinsight.org
Is HCl Polar or Nonpolar? Simple Explanation What's Insight Chlorine Electronegative Than Carbon 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to. since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon. Chlorine Electronegative Than Carbon.
From socratic.org
How are alkyl groups electron donating? + Example Chlorine Electronegative Than Carbon 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. examples include most covalent bonds. the two chlorine atoms share the pair of electrons in the single covalent bond. Chlorine Electronegative Than Carbon.
From www.pinterest.com
Electronegativity Definition and Trend Chlorine Electronegative Than Carbon since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. examples include most covalent bonds. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. the two chlorine atoms share the. Chlorine Electronegative Than Carbon.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence Chlorine Electronegative Than Carbon provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16. Chlorine Electronegative Than Carbon.
From www.alamy.com
Chlorine atomic structure Cut Out Stock Images & Pictures Alamy Chlorine Electronegative Than Carbon since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the. provided that you understand what happens when electronegative atoms like fluorine or chlorine are. Chlorine Electronegative Than Carbon.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Chlorine Electronegative Than Carbon provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Chlorine Electronegative Than Carbon.
From www.trentu.ca
PreChemistry Chlorine Electronegative Than Carbon the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. 119 rows electronegativity is a chemical property which describes how well an atom can attract. Chlorine Electronegative Than Carbon.
From nl.dreamstime.com
Electronegativity Periodieke Lijst Stock Illustratie Illustration of Chlorine Electronegative Than Carbon since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. the first scale of electronegativity was developed by linus pauling and on his scale chlorine has a value of 3.16 on a scale. the two chlorine atoms share the pair of electrons in the single. Chlorine Electronegative Than Carbon.